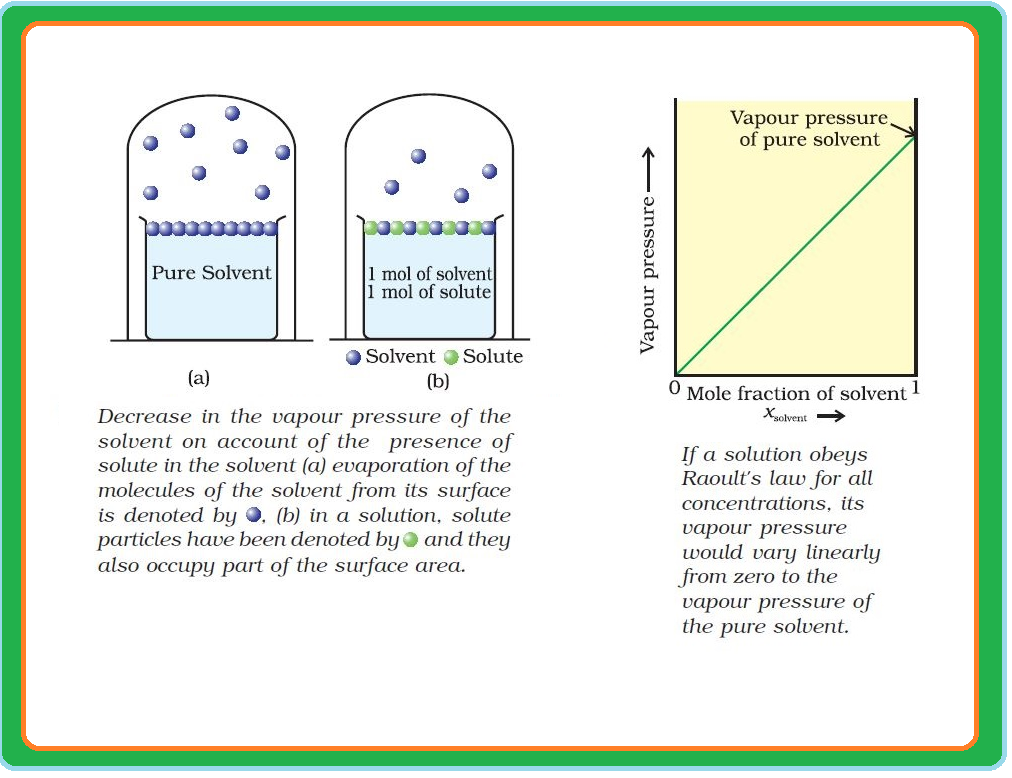
Roult's Law :
`=>` The French chemist, Francois Marte Raoult (1886) gave the quantitative relationship between mole fraction and partial pressure of a component in a solution.
`=>` This relationship is known as the Raoult’s law.
`color{green}("Statement") :` For a solution of volatile liquids, the partial vapour pressure of each component in the solution is directly proportional to its mole fraction.
Now, for component `1`
`color{red}(p_1 ∝ x_1)`
and `color{red}(p_1 = p_1^0 x_1)` .................(1)
where `p_1^0` is the vapour pressure of pure component `1` at the same temperature.
Similarly, for component `2`
`color{red}(p_2 = p_2^0 x_2)` ................(2)
where `p_2^0` represents the vapour pressure of the pure component `2`.
According to Dalton’s law of partial pressures, the total pressure (`p_text(total)`) over the solution phase in the container will be the sum of the partial pressures of the components of the solution and is given as :
`color{red}(p_text(total) = p_1 + p_2)` ................(3)
Substituting the values of `p_1` and `p_2`, we get
`color{red}(p_text(total) = x_1 p_1^0 + x_2 p_2^0)`
`color{red}(= (1 – x_2) p_1^0 + x_2 p_2^0)` ......................(4)
`color{red}(= p_1^0 + (p_2^0 – p_1^0) x_2)` .....................(5)
Following conclusions are drawn from equation (5) :
(i) Total vapour pressure over the solution can be related to the mole fraction of any one component.
(ii) Total vapour pressure over the solution varies linearly with the mole fraction of component `2`.
(iii) Depending on the vapour pressures of the pure components `1` and `2`, total vapour pressure over the solution decreases or increases with the increase of the mole fraction of component `1`.
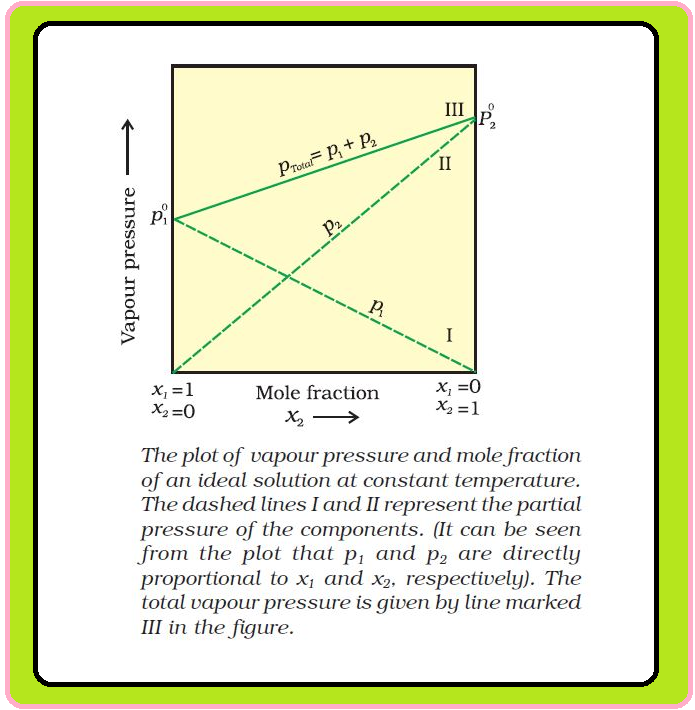
`=>` A plot of `p_1` or `p_2` versus the mole fractions `x_1` and `x_2` for a solution gives a linear plot as shown in Fig.
`=>` Lines (I and II) pass through the points and respectively when `x_1` and `x_2` equal unity.
`=>` Similarly the plot (line III) of `p_text(total)` versus `x_2` is also linear.
`=>` The minimum value of `p_text(total)` is `p_1^0` and the maximum value is `p_2^0`, assuming that component `1` is less volatile than component `2`, i.e., `p_1^0 < p_2^0`.
`=>` The composition of vapour phase in equilibrium with the solution is determined by the partial pressures of the components.
`=>` If `y_1` and `y_2` are the mole fractions of the components `1` and `2` respectively in the vapour phase then, using Dalton’s law of partial pressures :
`color{red}(p_1 = y_1 p_text(total))` ..................(6)
`color{red}(p_2 = y_2 p_text(total))` .....................(7)
In general
`color{red}(p_i = y_i p_text(total))`
`=>` This relationship is known as the Raoult’s law.
`color{green}("Statement") :` For a solution of volatile liquids, the partial vapour pressure of each component in the solution is directly proportional to its mole fraction.
Now, for component `1`
`color{red}(p_1 ∝ x_1)`
and `color{red}(p_1 = p_1^0 x_1)` .................(1)
where `p_1^0` is the vapour pressure of pure component `1` at the same temperature.
Similarly, for component `2`
`color{red}(p_2 = p_2^0 x_2)` ................(2)
where `p_2^0` represents the vapour pressure of the pure component `2`.
According to Dalton’s law of partial pressures, the total pressure (`p_text(total)`) over the solution phase in the container will be the sum of the partial pressures of the components of the solution and is given as :
`color{red}(p_text(total) = p_1 + p_2)` ................(3)
Substituting the values of `p_1` and `p_2`, we get
`color{red}(p_text(total) = x_1 p_1^0 + x_2 p_2^0)`
`color{red}(= (1 – x_2) p_1^0 + x_2 p_2^0)` ......................(4)
`color{red}(= p_1^0 + (p_2^0 – p_1^0) x_2)` .....................(5)
Following conclusions are drawn from equation (5) :
(i) Total vapour pressure over the solution can be related to the mole fraction of any one component.
(ii) Total vapour pressure over the solution varies linearly with the mole fraction of component `2`.
(iii) Depending on the vapour pressures of the pure components `1` and `2`, total vapour pressure over the solution decreases or increases with the increase of the mole fraction of component `1`.
`=>` A plot of `p_1` or `p_2` versus the mole fractions `x_1` and `x_2` for a solution gives a linear plot as shown in Fig.
`=>` Lines (I and II) pass through the points and respectively when `x_1` and `x_2` equal unity.
`=>` Similarly the plot (line III) of `p_text(total)` versus `x_2` is also linear.
`=>` The minimum value of `p_text(total)` is `p_1^0` and the maximum value is `p_2^0`, assuming that component `1` is less volatile than component `2`, i.e., `p_1^0 < p_2^0`.
`=>` The composition of vapour phase in equilibrium with the solution is determined by the partial pressures of the components.
`=>` If `y_1` and `y_2` are the mole fractions of the components `1` and `2` respectively in the vapour phase then, using Dalton’s law of partial pressures :
`color{red}(p_1 = y_1 p_text(total))` ..................(6)
`color{red}(p_2 = y_2 p_text(total))` .....................(7)
In general
`color{red}(p_i = y_i p_text(total))`